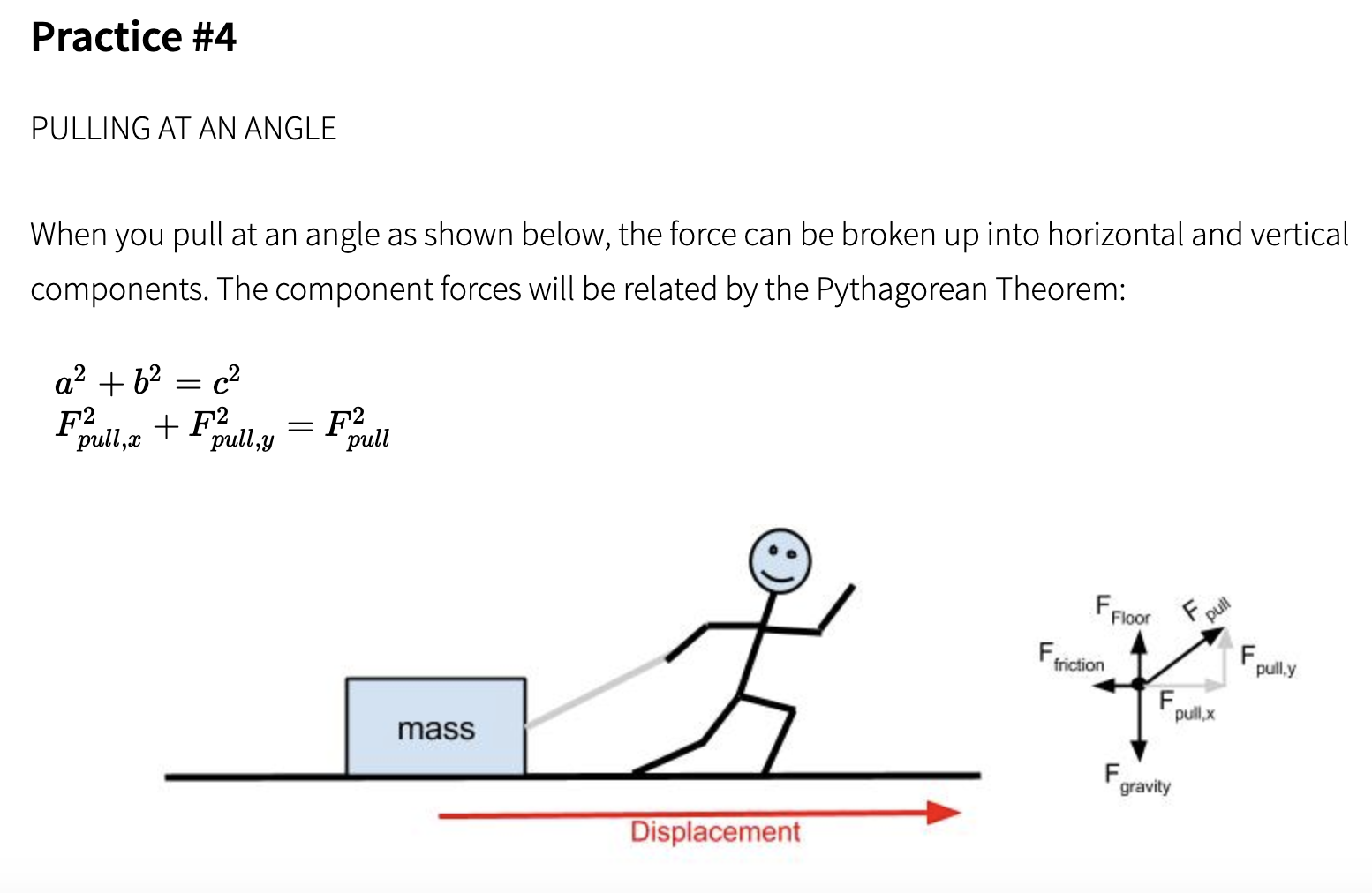
Work done is defined in such a way that it involves both Force applied on the body and the displacement of the body. Consider a block placed on a frictionless horizontal floor. This block is acted upon by a constant force F. Action of this force is to move the body through a distance d in a straight line in the direction of force. Practice: Calculating work done by a force. This is the currently selected item. Introduction to work review. Calculating work from force vs. Position graphs. Introduction to work review. Introduction to work review. Our mission is to provide a free, world-class education to anyone, anywhere. Work done is the force multiplied with the distance moved by the force - and can be expressed as W = F s (1).
Start free. Go premium when you’re ready.

Free for up to 3 users.
14 day free trial for 4 or more users. No credit card required.
How many people in your company?
OKR Coaching & Support
Your personal dedicated OKR Coach
Live Chat support
Account set up and onboarding
Team trainings
Live Webinars
Personalized quarterly OKR reviews
Unlimited sessions customized to your needs
Core Features
OKRs and weekly planning
Conversations, Feedback, and Recognition (CFR)
Clear dashboards and progress breakdowns by user
True OKR alignment (top down or bottom up)
Company TV Dashboards and Custom Reports
Integrations
Slack & Microsoft Teams
Jira & Asana
Basecamp
Google Tasks
Over 1,500+ integrations through Zapier
SSO possibility
Custom API access
Pricing FAQ
Is it really free for 3 users?Yes, free for up to three users. The account is 100% fully functional and you have access to all included features.
How long is our Weekdone data kept?Your data remains accessible while you use Weekdone. If you stop using Weekdone, you can request to have your data deleted and we will remove all data from the Weekdone account. We comply with GDPR data protection regulations for the handling and anonymization of personal data.
What happens when I add more than 3 users?Once you have more than 3 users, you will enter a 14 day free trial. You can add as many users as you like during your trial. After your 14 days you can enter a paid subscription to continue usage.
Are all the features actually included?Yes! All are included. You can read about all the features here. The free package for up to 3 users also contains all features.
How is my personal data handled?We are fully GDPR compliant and our data is stored in the EU. We can provide a DPA on request. Read more on our security.
Is individual training & support included?Yes! We know that transitioning to OKRs can be challenging. For that, we provide live help and training in addition to our live chars and instruction materials to personally get you and your company onboarded. Read about everything included here.
Do you have materials to help support and onboard our users?We have a full learning center for leaders, managers, and users of Weekdone. Support is not just limited to admins, but you can also schedule a live web conference call or training with our experienced OKR coaches or onboarding staff for your entire team.
Does the price change as we grow?Don’t fear surprise price hikes, we have per user pricing which decreases per user as you grow. You can also change from a monthly to annual plan at any time to save 20%, two months free!
What if I need some quick help?We have a live support accessible for all users. Feel free to reach out at any time. For any additional questions email us at sales@weekdone.com or send us a message on our Support Chat below.
Thermodynamics is a branch of physicswhich deals with the energy and work of a system.Thermodynamics deals only with thelarge scale responseof a system which we can observeand measure in experiments. In aerodynamics, we are mostinterested in the thermodynamics ofhigh speed flows, and inpropulsion systems which produce thrust byaccelerating a gas. To understand how thrust is created, it is usefulto study the basic thermodynamics of gases.
The state of a gas is determined by thevalues of certain measurable propertieslike the pressure,temperature,andvolumewhich the gas occupies. The values of these variables andthe state of the gas can be changed. On this figure we show a gasconfined in a blue jar in two different states. On the left, in State1, the gas is at a higher pressure and occupies a smaller volume thanin State 2, at the right. We can represent the state of the gas on a graphof pressure versus volume, which iscalled ap-V diagramas shown at the right.To change the state of the gas from State 1 toState 2, we must change the conditions in the jar, either by heatingthe gas, or physically changingthe volume by moving a piston, or by changing the pressure by adding or removingweights from the piston. In some of these changes, we do workon, or have work done by the gas, in other changes we add, orremove heat. Thermodynamics helps us determine the amount of workand the amount of heat necessary to change the state of the gas.Notice that in this example we have a fixed mass of gas. We cantherefore plot either thephysical volumeor thespecific volume, volume divided by mass,since the change is the same for a constant mass. On the figure,we use physical volume.
Scientists define workW to be the productof force F acting through a distance s :
W = F * s
For a gas, work is the product ofthe pressure p and the volume V during a change of volume.
W = p * V
We can do aquick units check to see that pressure force / area times volumearea * length gives units of force times length which are the units of work

W = (force / area) * (area * length) = force * length
In the metric system the unit of work is the Joule, in the English systemthe unit is the foot-pound.In general, during a change of state both the volumeand the pressure change. So it is more correct to define the work asthe integrated, or summed variable pressure times the change of volumefrom State 1 to State 2. If we use the symbol S [ ] ds for integral, then:
W = S [p] dV

On a graphof pressure versus volume, the work is the area under the curve thatdescribes how the state is changed from State 1 to State 2.
As mentioned above, there are several options for changing the state ofa gas from one state to another. So we might expect that the amount ofwork done on, or by a gas could be different depending on exactly how the stateis changed. As an example, on the graph on the figure, we show a curvedblack line from State 1 to State 2 of our confined gas.This line represents a change brought about by removing weightsand decreasing the pressure and allowing the volume to adjust accordingto Boyle's law with no heat addition. The lineis curved and the amount of work done on the gas is shown by the redshaded area below this curve. We could, however, move from State 1to State 2 by holdingthe pressure constant and increasing the volume byheating the gas using Charles' law. Theresulting change in state proceeds from State 1 to an intermediateState 'a' on the graph. State 'a' is at the same pressure as State 1,but at a different volume. If we then remove the weights, holding aconstant volume, we proceed on to State 2. The work done in thisprocess is shown by the yellow shaded area. Using eitherprocess we change the state of the gas from State 1 to State2. But the work forthe constant pressure process is greater than the work for the curvedline process. The work done by a gas not only depends on the initialand final states of the gas but also on the process used to changethe state. Different processes can produce the same state, butproduce different amounts of work.
Notice that not only does the work done by the gas depend on the process, butalso the heat transferred to the gas. In the first process, the curved line fromState 1 to State2, no heat was transferred to the gas; the process was adiabatic.But in the second process, the straight line from State 1 to State 'a' and thentoState 2, heat was transferred to the gas during the constant pressure process.The heat transferred to a gas not only depends on the initialand final states of the gas but also on the process used to changethe state.
Activities:Guided Tours
- Thermodynamics:
Navigation ..
Work Done Inc
Work Done By Friction
- Beginner's Guide Home Page

Comments are closed.