Main Difference – Carbon 12 vs Carbon 14
Some elements can exist in different forms known as isotopes. Isotopes of an element contain the same number of electrons and protons, but a different number of neutrons. Hence, even if it has the same element, their mass is different. The mass number of an element is the sum of neutrons and protons in its nucleus. Thus, isotopes are denoted by their mass number. For example, carbon is an element that exists in three forms; in other words, carbon has three isotopes: carbon-12, carbon-13 and carbon-14. The mass number of carbon-12 is twelve as it contains 6 neutrons and 6 protons. Likewise, carbon-13 isotope contains 7 neutrons and 6 protons, while carbon-14 isotope contains 8 neutrons and 6 protons. Most elements with isotopes have one major isotope present abundantly in nature while the rest of the isotopes present in very small proportions. Hence, when considering the relative atomic mass of an element, it is usually assumed that the relative mass number is equal to the mass number of the major or abundantly existing isotope. The main difference between carbon 12 and carbon 14 isotopes is their stability; carbon 12 isotope is more stable than carbon 14.
- 3.2 Carbon Carbon (C, atomic number 6) occurs in nature predominantly as the stable isotopes carbon-12 (98.89%) and carbon-13 (1.1%). Its most important radioactive isotope is carbon-14, a weak beta-emitter having a half-life of 5730 years. Carbon-14 is formed naturally in the upper atmosphere by the action of cosmic rays on nitrogen.
- Diagram showing the nuclear composition and electron configuration of an atom of carbon-12 (atomic number: 6), the most common isotope of the element carbon (C). The nucleus consists of 6 protons (red) and 6 neutrons (blue). Six electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
The atomic mass unit is defined as 1/12th the mass of a carbon-12 atom. This is not correct. The unified atomic mass unit u is defined as 1/12th the mass of a carbon-12 atom. The atomic mass unit (amu) is defined as 1/16th the mass of the oxigen-16 isotope (physics) or 1/16th of the (average) mass of an oxigen atom (chemists).
Key Areas Covered
1. What is Carbon 12
– Definition, Structure, Properties
2. What is Carbon 14
– Definition, Structure, Properties
3. What is the difference between Carbon 12 and Carbon 14
– Comparison of Key Differences
Key Terms: Isotopes, Carbon, Carbon Isotopes, Mass Number, Neutrons, Carbon 12, Carbon 14, Radioactivity, Avogadro Constant
Atomic Number Of Carbon 12
What is Carbon 12
Carbon-12 isotope is the most abundant carbon isotope that makes up of about 98.89% of all naturally occurring carbon. It is found in all biological systems. Carbon-12 atom contains 6 neutrons and 6 protons in its nucleus. Carbon-12 isotope is stable and not radioactive. Therefore, it does not decay, unlike carbon-14. Carbon-12 atom is used to define the relative atomic mass scale, where the masses of other atoms are compared with the mass of an atom of the carbon-12 isotope. Here, carbon-12 is taken as the standard atom. The relative atomic mass (RAM) of elements is shown in the periodic table. Just like the RAM, the mole is based on the carbon-12 isotope. The number of atoms in 12.00 g of carbon-12 is taken as the standard to define the mole. The exact number of atoms in 12 g of carbon-12 is found to be 6.02 x 1023. An Italian Chemist, Amedeo Avogadro discovered this number in the nineteenth century. This number is defined as the Avogadro constant. The unit of Avogadro constant is mol-1.
What is Carbon 14
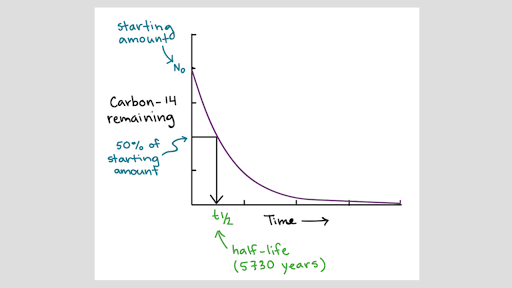
Carbon-14 is the unstable isotope of carbon and contains 8 neutrons and 6 protons; hence the mass number is 14. Unlike other isotopes of carbon, carbon-14 is radioactive; thus, it decays with time. Carbon-14 isotope makes up of about less than 0.01% of all naturally occurring carbon. The decaying action of carbon-14 is spontaneous. Carbon-14 decays to form nitrogen-14 atom. Organisms gain carbon-14 during photosynthesis or while eating organic matter. When the organism dies, it stops taking carbon-14 sources. With that, carbon-14 starts to decay and becomes half of its initial amount in about 5730 years, which is called the half-life of carbon-14. The remaining amount of carbon-14 can be measured and compared to the amount present in most living specimens. By doing that, scientists can decide the age of fossils using carbon-14. However, carbon-14 is applied to fossils that are older than 50,000 years because the radioactivity of carbon-14 isotope becomes very slow after 50,000 years.
Figure 1: Formation and decay of Carbon-14
Difference Between Carbon 12 and Carbon 14
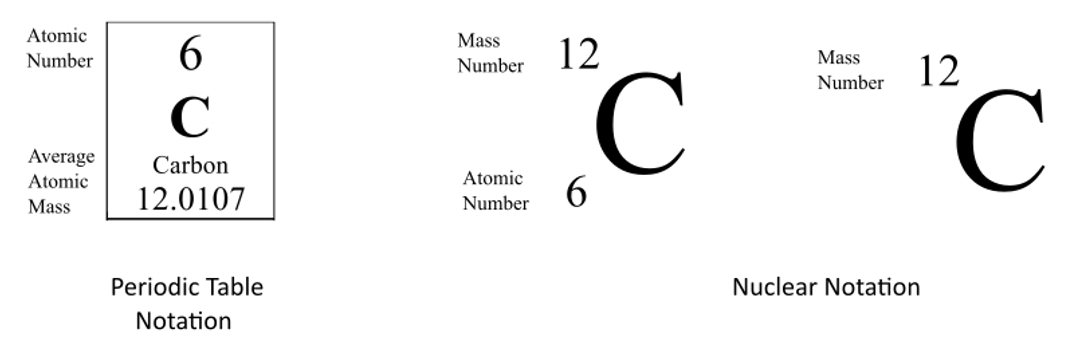
Mass Number
Carbon-12: Mass number of Carbon-12 is twelve.
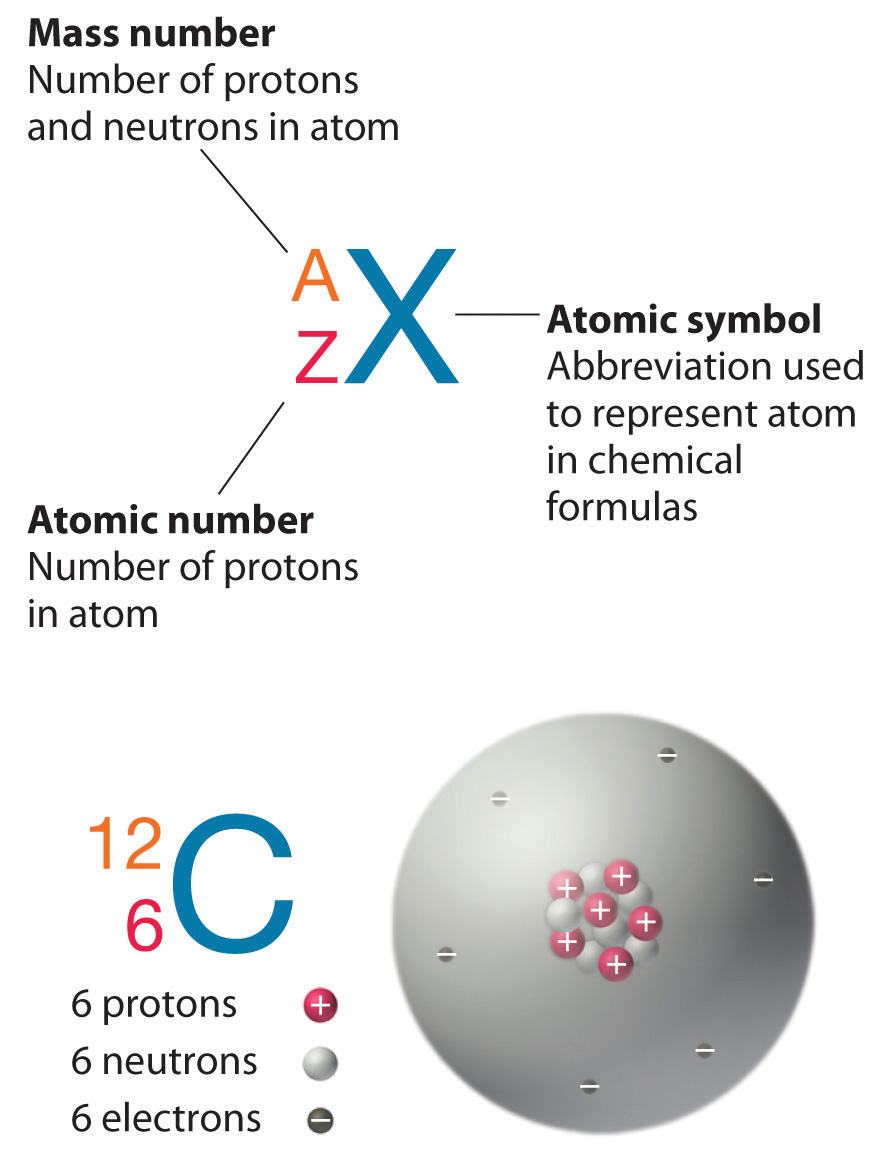
Carbon-14: Mass number of Carbon-14 is fourteen.
Number of Neutrons in an Atom
Carbon-12:Carbon-12 has six neutrons.
Carbon-14:Carbon-14 has eight neutrons.
Stability
Carbon-12:Carbon-12 is stable.
Carbon-14: Carbon-14 is unstable.
Radioactivity
Carbon-12:Carbon-12 is not radioactive.

Carbon-14: Carbon-14 is radioactive.
Application
Carbon-12:Carbon-12 is the building block of all biological systems.
Carbon-14: Carbon-14 is used to measure the age of fossils that dates back to 50,000 years.
Isotope Distribution
Carbon-12: Carbon-12 is found in 99% of all naturally occurring carbons.
Carbon-14: Carbon-124 makes up less than 0.01% of all naturally occurring carbons.
Conclusion
Carbon-12 and carbon-14 are two types of isotopes of carbon. Carbon-12 is the most abundant isotope of carbon and is stable due to the absence of radioactivity. However, carbon-14 is not stable due to its radioactivity. Because of that carbon-14 is found very rarely in biological systems. This is the difference between carbon-12 and carbon-14.
Reference:
Carbon Number Of Neutrons
1.Breithaupt, Jim. Physics (Palgrave Foundations Series). N.p.: Palgrave Macmillan;, 2010. Print.
2.Knorr, Susan. Learning about atoms. Greensboro, NC: Mark Twain Media, 2004. Print.
Image Courtesy:
1.”Carbon 14 formation and decay” By C14_methode_physikalische_grundlagen.svg: Sgbeerderivative work: NikNaks talk – gallery – wikipedia – C14_methode_physikalische_grundlagen.svg (CC BY-SA 3.0) via Commons Wikimedia


Comments are closed.